Efficacy of OT-82 in preclinical models (summary)
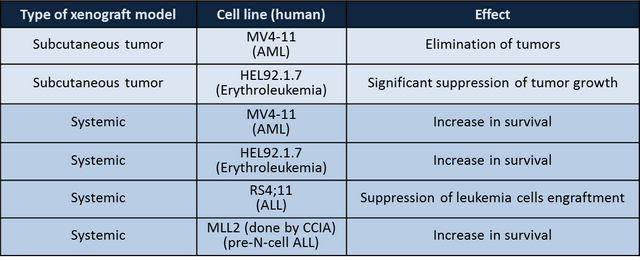
OT-82 has demonstrated efficacy against:
- Systemic and subcutaneous xenografts of human leukemia (3) , lymphoma (1) and multiple myeloma (1)
- Systemic preclinical models based on patient-derived cells from pediatric ALL patients (at Children Cancer Institute of Australia, Sydney)
OT-82 Mechanism of Action
NAMPT catalyzes conversion of nicotinamide to nicotinamide mononucleotide (NMN), a precursor of NAD. That determines its key role in bioenergetics of the cell and in the activity of NAD-dependent proteins, such as sirtuins, PARPS, etc. NAMPT exists in intracellular and extracellular forms. In the cell NAMPT is present in the cytoplasm and nucleus. Its phosphorylated form has 100-times higher activity. The role of other post-translational modifications is not defined yet.
Majority of tumor cells are very sensitive to NAMPT inhibition, due to a higher demand for NAD+ and ATP. Out of all malignancies, leukemia cells are those the most dependent on activity of metabolic pathways utilizing NAD+ [Ying-Hua Wang, Cell 158, 2014, 1309-1323]
OT-82 Toxicology profile
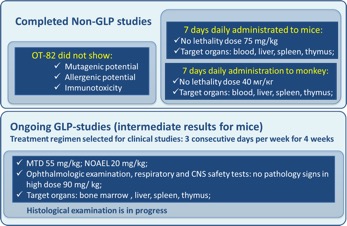
In 4 week GLP study in mice, NO DETECTABLE RETINAL TOXICITY was found.
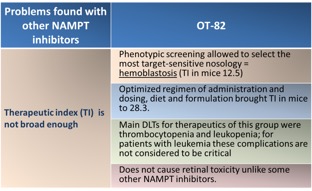
Competitive landscape and OT-82 advantage
In addition, although NAMPT inhibition potency of OT-82 is similar to other known inhibitors in vitro, it demonstrated higher potency in an in vivo pre-B cells acute lymphoblastic leukemia (ALL) xenografts. This test was run at the Children’s Cancer Institute Australia for Medical Research (CCIA) according to the NCI-supported Preclinical Testing Program protocol established for evaluation of new agents against solid tumors and leukemia models.
OT-82 Development timeline
